Auto alerts prioritized as warning and critical alerts, based on equipment failure or procedural error. Easy monitoring of multiple alarms
Auto alerts prioritized as warning and critical alerts, based on equipment failure or procedural error. Easy monitoring of multiple alarms
The roles of alarms in the life sciences industry cannot be underestimated. Given how influential they are on product quality, they are monitored and given close attention. As a result, many companies have established clear-cut procedures for managing their alarm systems.
The reality of Pharma 4.0 in this fast-paced and scientifically advanced age has rendered manual monitoring of alarms largely inefficient. The consequences of the lapses and shortcomings of a manually monitored alarm could prompt 483 observations from the US FDA. Pharmaceutical, biotech, biosimilar, and API manufacturing industries are some of the life sciences organizations that need to put in place adequate facilities and systems that would aid their alarm management.
Alarm management systems are mentioned in EPA, OSHA, and cGMP documents.
Organizations set up alarm systems to alert employees in case of equipment failure or a procedural error. It helps organizations in reducing process inconsistencies or problems that may need rework or even result in product loss. So, it is a system meant to notify and give reactive procedures for unfavorable circumstances, as well as process conditions and equipment failures that are beyond tolerance.
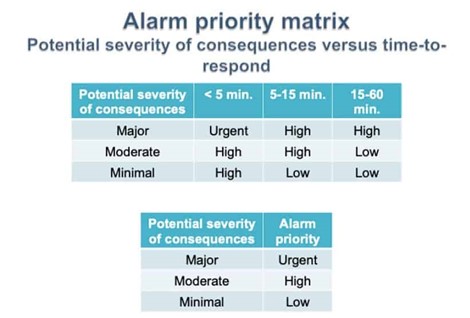
Alarm kinds are often categorized according to the type of plant they are associated with. Most companies have at least two distinct alarm kinds. These two types of alarms are warning and critical, respectively.
Any disruptions in the plant system cause a departure from typical operating conditions in biotech companies. It suggests that the system is operating outside of its regular operation range. Environmental, product quality and safety procedures may all be improved by implementing an efficient alarm system that notifies the company when the conditions become abnormal. These alarms are often referred to as warning alarms.
Deviations will increase if warning alarms are not appropriately attended to. The system then goes into critical mode alarm when they reach Proven Acceptance Range (PAR). This alarm requires a prompt response from the personnel in charge in order to quickly arrest the situation and reduce damage.
For instance, stability chambers, freezers, and incubators are examples of equipment used by pharmaceutical and biotech organizations. These pieces of equipment keep products in a regulated environment. Any change in temperature can potentially put product quality and the safety of the personnel and equipment at risk.
Using an alert system helps detect abnormal temperatures on time so that quick action can be taken to mitigate the effects of the temperature change.
Alarm Management is critical in analyzing and controlling operational hazards associated with manufacturing facilities in the Life Sciences industry. Knowing fully well that poor alarm functionality or an unreliable system could jeopardize your plant’s productivity and safety requirements, it is imperative to have an effective alarm system in place.
Recently, the United States Food and Drug Administration (FDA) discovered that some organizations are not adhering to alarm systems through assessments. As a result, companies have received warning letters requiring them to improve their alarm management systems in order to meet statutory requirements.
Among these observations are:
GMP Guidelines for Alarm Management Systems
FDA’s guidance for alarm management can be observed in two of its guidelines
Two of the FDA’s guidelines on alarm management provide recommendations for alarm management.
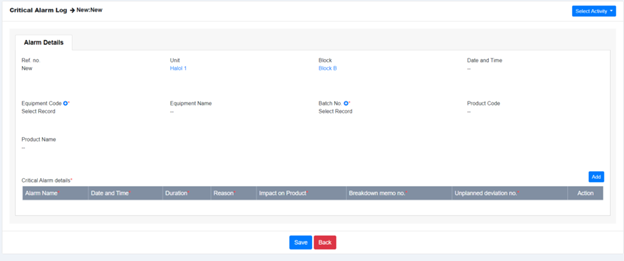
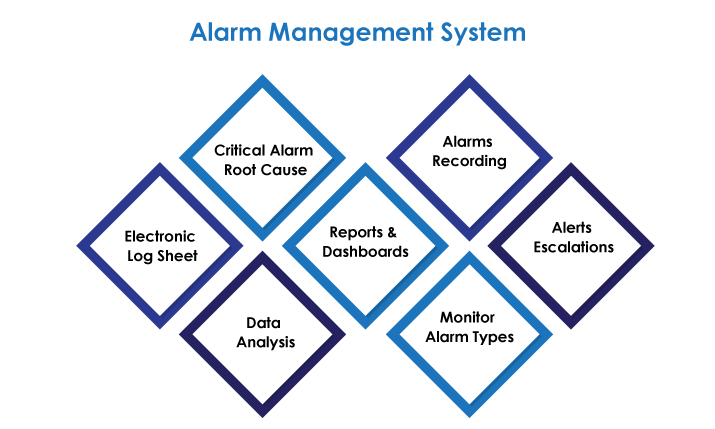
AmpleLogic Alarm Recording Software helps you to document the following:
This alarm recording software of ours helps in the monitoring of various alarms, their main sources, and possible corrective or preventive measures to be taken to address the problem.
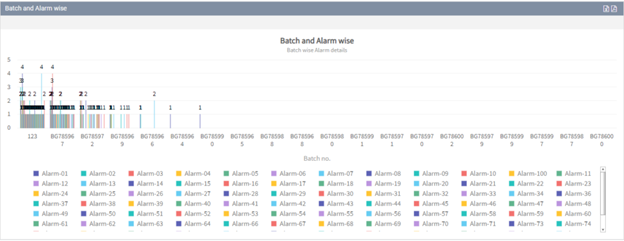
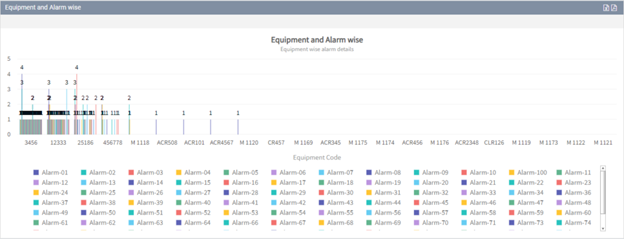
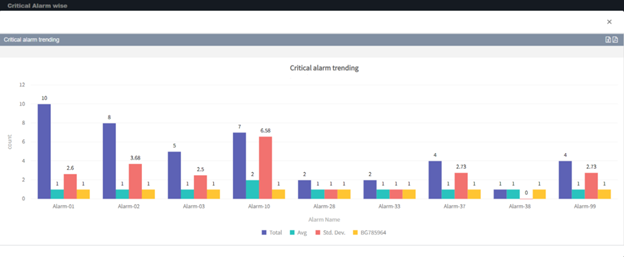
You can easily analyze alarm data for the equipment using our alarm tracking software to monitor the effectiveness, dangers, and deviations that may arise in each piece of equipment in the plant.
Create alarm reports to assist the organization in giving helpful insights that can be used to improve the systems in the process
AmpleLogic Critical Alarm Tracking Software will also comply with electronic Record standards defined by TGA, CDSCO, HEALTH CANADA, MCC, ANVISA, EMEA, SFDA, NAFDAC, MEDSAFE,MHLW, MCAZ, SWISSMEDIC, KFDA and MoH.
Please contact us if you would like to learn more about how AmpleLogic Alarm Management Solution can increase the efficiency of your organization.