Harmonize voluminous logbooks with our hassle-free Elogs software to revolutionize data capture, maintain record-keeping and compliance through efficient electronic logging
Electronic Logbook (eLogbook) Software for Pharmaceuticals
AmpleLogic Electronic Logbook is a web-based software or platform that records general production requirements and keeps track of Area and Equipment operational usage, Packing, Cleaning, Break Down, Clearance, and Preventive Maintenance, Fogging and Defogging Log, Granulation, Calibration, Equipment Usage, Stability Schedule, Standards Usage, Service Log, Dispensing, Production, Chemical Usage Log, and many other equipment details log.
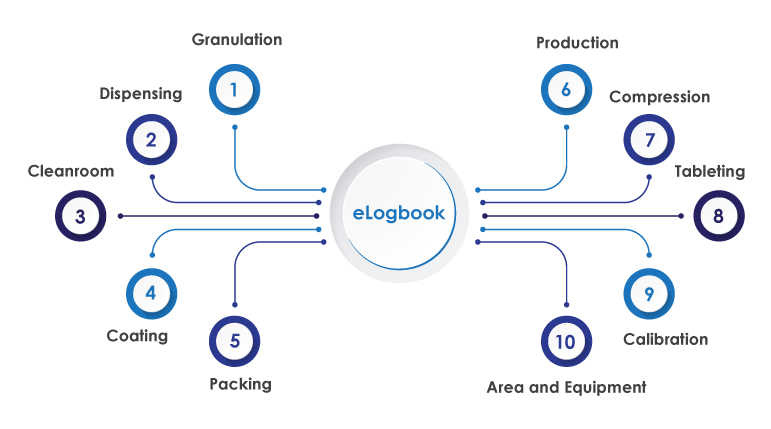
This eLogbook Software is designed to assist users in converting manual paper forms to electronic equivalents. Additionally, the logbook software guarantees that details or log are entered accurately and on time, and that they may be validated, reviewed, and authorized via approval workflows.
AmpleLogic Equipment Usage Logbook Software is compliant with FDA regulations governing electronic records and signatures, including 21 CFR Part 11. This solution also complies with other regulatory standards such as EU Annex 11, MHRA, GAMP, Good Manufacturing Practice (GMP), Standard Operating Procedures (SOP’s), and ISO. The software was created having in mind the requirements of major regulatory authorities, and to better serve our clients
The AmpleLogic eLogbook Software/Electronic Logbook guarantees that information is disseminated throughout the organization’s essential operational departments, including the quality, production, planning, and maintenance departments, and they serve to bring clarity and transparency to the entire organization’s production and QC lab activities.
AmpleLogic’s Electronic Logbook Software helps in the configuration of different types of logbooks.
AREA AND EQUIPMENT LOGBOOK |
|
|---|---|
| Equipment Usage log | Boiler Operation log |
| Environment Condition | HPLC Usage log |
| Calibration Log | AHU Parameter Monitoring |
| Chiller Operation log | HEPA Filter Replacement |
| Purified Water Distribution | Air Compressor Monitoring |
| Semi-Micro Balance log | TDS Analyzer log |
| Pressure Monitoring | Melting Point Apparatus |
| Electrical Substation Operation | Maintenance log |
QUALITY CONTROL LOGBOOK |
|
|---|---|
| Sample Registration log | Stability Protocol log |
| Chemical initiation log | Chemical Booking log |
| Sample Management log | Standard log |
| Impurity Standard log | Working standard log |
| Reference Standard log | Laboratory standard log |
| Column Usage log | Incubation log |
| Experiment template log | Sample Withdrawal log |
| Pilot experiment log | GC Usage log |
DOCUMENTATION LOGBOOK |
|
|---|---|
| Document Issuance log | Quality observation log |
| Request Issuance, Retrieval log | Extension request for OOC log |
| Training Material Issuance | Batch Detail log |
| Training attendance log | Packing Material log |
CLEANING AND SANITATION LOGBOOK |
|
|---|---|
| Weekly Drain Point sanitation | Accessories Cleaning Log |
| In-Process Container Cleaning | Sequential Log |
| Portable equipment log | Daily cleaning or sanitation |
Challenges with manual paper-based logbooks
Lifesciences companies like Pharmaceutical, Biotech, Contract Research Organization (CRO), Medical Devices and Contract Development and Manufacturing Organization (CDMO), API (Active Pharmaceutical Ingredient) Manufacturers and other drug manufacturing companies use manual paper-based logbooks, they face numerous recurring impediments. With a manual paper-based system, it is difficult to record specifics like equipment calibration, stability schedules, granulation, packing, tableting, area cleaning, maintenance, weighing and dispensing, and equipment usage logs. Manual data entry in the logbook has been shown to decrease productivity, contain unnecessary information, inaccuracies, and contain incorrect estimates of production quantities, deviation, and so on.
The usage of a paper-based logbook has a history of causing compliance problems. Additionally, it affects operational processes, which are prone to inaccuracy when capturing the contents of individual logs with user signatures, date and time stamps, test and product specifications, and batch numbers. The validation process is also complicated by the fact that each batch’s lot numbers are handled in multiple places.
Additionally, it is essential to evaluate logbook entries regularly to ensure that data has been appropriately compiled over time following SOP standards. Using a different logbook for each phase makes the process more time-consuming, resulting in an accumulation of many logbooks. This has a knock-on effect on document verification.
Documents in the logbook may need to be corrected. A record of the procedure is retained, together with a signed and dated statement from all parties involved, explaining why a revision was made. When these records are transferred to different departments, tracing or tracking them manually becomes complex and demanding.
We at AmpleLogic developed the Electronic Logbook (eLogs) as a solution to the problems encountered during data entry and the capture of all usage log details across facilities to avoid these issues. This is purposely created for FDA-regulated (GMP/cGMP) manufacturing facilities to overcome the numerous difficulties encountered in the various departments of an organization during log entry.
Key Features of AmpleLogic Electronic Logbook System(eLogs)
- It is compliant with 21 CFR Part 11 standards
- Defining a framework for regular log entries across your organization guarantees that you are prepared for an audit or inspection when the time comes
- AmpleLogic Pharma Logbook Solution keeps track of Calibration, Maintenance Service Logs, Equipment Cleaning Logs, Packing Material Logs, Out of Calibration Investigation Logs, OOS Investigation Extension Logs, and Equipment Calibration Logs, among other things
- The Equipment Logbook Application also includes a function that keeps track of Area and Equipment cleaning, usage records of standards, columns, chemicals, stability schedules, ANDA and DMF Tracking Logs, and so on
- Additionally, all of your log data are displayed in calendar format, allowing you to see schedules of plans and when they are due
- By using Digital Logbook Software, Log Reports can be configured by users to meet a variety of needs, including management and compliance. The E-Log Management Software lets you read, print, or save reports as a pdf, CSV, or XML file
- The eLogs System creates reports for both operational and non-operational operations, such as Area Usage, Equipment Usage, Checklist Issuance & Execution, Cleaning and Sanitation Logs, and others
- The Electronic Log Management Software will automatically send notifications and escalate to ensure that specific logs such as equipment logs, calibration logs, HPLC Column logs, maintenance logs, cleaning, stability schedule logs, chemical usage logs, quality observation logs, training attendance logs, and other instrument logs are current and have been authenticated and assessed on time
- Users and groups in the department are notified by Equipment Usage Logbook Software when usage logs expire
- It implements the procedures for log recording, verification, review, and authorization as specified in SOPs and other standards
- AmpleLogic Electronic Logbook Solution manages document issuance, training attendance, standard operating procedures (SOP) issuance and retrieval, training log issuance register, and training record destruction
- SOP numbering register, quality observations, SOP issuance register for uncontrolled copies, master SOP index, a master index, request issue, and retrieval of bound books are all managed by our paperless Logbook Software
- Full visibility for usage logs and authentic validation condition of equipment, devices, and manufacturing facilities will be ensured by AmpleLogic Pharmaceutical Electronic Logbook Software
- You can upload and download logbook entries saved in one of the authorized formats using the e-Log Management Software. Additionally, for your convenience, we have included a template that may be customized to fit your specific needs
- This eLogs solution is compatible with machines, programmable logic controllers, historical databases, SAP, and other third-party applications
Benefits of AmpleLogic Logbook Software
- It affords sufficient time and attention to be devoted to business and other resources rather than data entry and correction of errors, which are addressed by the program
- This Electronic Logbook system enables continuous monitoring of the entire production process
- The Electronic Equipment Logbook system makes it possible to keep tabs on the entire operation as it’s taking place.
- This eLogbook software automates the entire production process, eradicating the need for paper and manual data entry
- AmpleLogic’s Paperless Logbook Software, eliminates paper-based logbooks completely, save hundreds of tedious man hours in generating, reviewing, tracking and approving multiple logs
- The paperless logbook solution allows users to work in multiple languages
- Because everything is already saved in the system, printing and binding is not needed
- Electronic Logbooks ensure the integrity of the data they contain
- There is no risk of misplacing or losing logbooks
Pharmaceutical companies highly value AmpleLogic Electronic Logbook (eLogs) for capturing paperwork and preserving records in electronic form. The system is very beneficial to Quality Control (QC) teams.
The AmpleLogic Digital Logbook Software (eLog) is widely accepted by pharmaceutical firms to capture paperwork and maintain records electronically. This system is being used by the Quality Control (QC) laboratories’ R&D team to retain their GMP (Good Manufacturing Practices) logbooks.
AmpleLogic eLogbook software conforms with TGA, CDSCO, HEALTH CANADA, MCC, ANVISA, EMEA, SFDA, NAFDAC, MEDSAFE, MHLW, MCAZ, SWISSMEDIC, KFDA, and MoH electronic record requirements.
AmpleLogic Equipment Log Management Software meets the needs of other Life Sciences Industries like :
- Biologics
- Medical Devices
- Contract Development and Manufacturing Organization (CDMO)
- Chemical Industry
- Contract Manufacturing Organization (CMO)
- API (Active Pharmaceutical Ingredient) Manufacturers
- Generics
- General Manufacturing
- Contract Research Organization (CRO)
- Food & Beverages
Please contact us if you would like to learn more about how AmpleLogic Electronic Logbook Management Software (eLogbook) may help your organization
