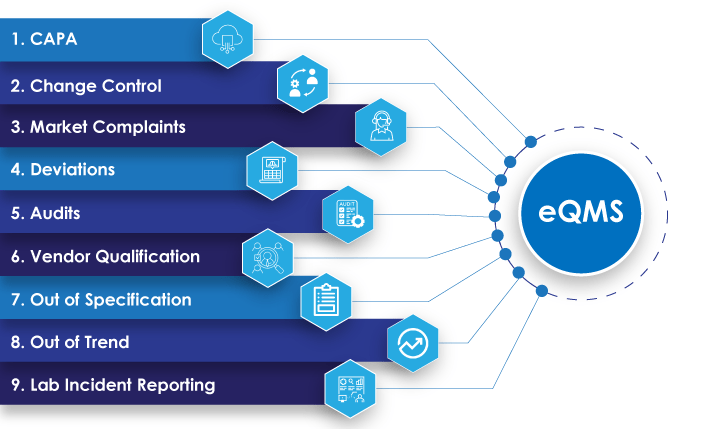
Ensure quality excellence in the pharmaceutical industry with our end-to-end software focusing on compliance, risk management, and continuous improvement
Part of the standards established by international regulatory authorities such as the FDA, MHRA, and EU Annex 11 is for pharmaceutical and life sciences companies to create and design an Effective Vendor Qualification Management system that checks vendors and third parties who patronize their products globally.